Featured Titles
CARBON PARADOX
THE CARBON PARADOX:
Why are we here?
Why are we alone?
And how long have we got to live?

Explanatory notes and references for the YouTube video ‘Carbon Paradox’:
The Fermi Paradox:
In 1950 Italian physicist Enrico Fermi asked several of his colleagues at Los Alamos: “Where is everybody”? There are various accounts of the question possibly indicating the conversation with Emil Konopinski, Edward Teller and Herbert York – compiled by Eric M. Jones – occurred separately to a conversation with Leo Szilard wherein Szilard quipped that aliens were already here, and they were called Hungarians. (There were a number of Hungarians working at Los Alamos, joking nicknamed the ‘Martians’.)
“… he went on to conclude that the reason that we hadn’t been visited might be that interstellar flight is impossible, or, if it is possible, always judged to be not worth the effort, or technological civilization doesn’t last long enough for it to happen.” – E. M. Jones (1985) “Where is everybody?” An account of Fermi’s question, Los Alamos National Laboratory
Read more: ‘The Fermi Paradox Is Not Fermi’s, and It Is Not a Paradox’ – Scientific American
The (Modified) Drake Equation:
Around this time several scientists – many with links to Los Alamos, like professor of physics at MIT Phillip Morrison – published theories regarding the search for extraterrestrial life. In 1961 astronomer Frank Drake helped convene a meeting at the Green Bank Observatory, where he created the ‘Drake Equation’ as a form of agenda for the meeting, to estimate the number of intelligent civilizations in our galaxy. Original estimates by Drake and his colleagues deduced that somewhere between a billion and 100 million extraterrestrial civilizations should exist in the Milky Way alone.
In 2016, Dr. Adam Frank from the University of Rochester and Dr. Woodruff “Woody” Sullivan from the University of Washington modified Drake’s Equation to consider the number of alien civilizations ‘ever’. They concluded the probability that humans are alone in the Milky Way to be 1-in-60 billion.
Drake, Frank and Sullivan all added more weight to Fermi’s original question, “Where is everybody”?
Bracewell-von Neumann Probes:
Mathematician John von Neumann was one of the Hungarian ‘Martians’ at Los Alamos joked about by Leo Szilard (above). Von Neumann worked on the concept of self-replicating ‘automata’ throughout the 40s and 50s. Oddly, he did not comment specifically on their use in space exploration. Rather, it was in 1960 that professor of Electrical Engineering at Stanford University, Ronald N. Bracewell, proposed the use on von Neumann’s automata in space exploration. Later again, it was mathematical physicist and cosmologist Frank Tipler who calculated (in his 1981 paper titled ‘Extraterrestrial Beings Do Not Exist’ in the Quarterly Journal of the Royal Astronomical Society, volume 21, pages 267-281) that it would take these drones a period of 4 million years to explore the entire known universe. In this, Bracewell and Tipler added exponentially to the Drake-Frank-Sullivan estimates of the probability of encountering alien civilisation since estimates were no longer confined to the Milky Way.
Further, Tipler was rebutted by Carl Sagan and William Newman 2 years later. Published in the same journal, Sagan and Newman showed Bracewell-von Neumann Probes would replicate exponentially and therefore universal exploration could occur in half the time estimated by Tipler. (See ‘The Solipsist Approach to Extraterrestrial Intelligence’, volume 24, page 113). In other words, the Universe could have been explored 7000 times over since the Big Bang.
When Frank and Sullivan ran their modified Drake calculation for the entire Universe, the result was a 1-in-10-billion-trillion probability that human’s are the first technological life form.
If you multiply the calculations by Sagan and Frank/Sullivan it is possible to demonstrate that we should have encountered evidence of many trillions of other life forms, with additional Bracewell-von Neumann Probes being encountered in constant waves of interstellar activity.
Again, much more so, “Where is everybody”?
The Gaian Bottleneck Hypothesis:
Astrobiologists Aditya Chopra and Charley Lineweaver at the Australian National University proposed the ‘Gaian Bottleneck Hypothesis’ in their January 2016 paper ‘The Case for a Gaian Bottleneck: The Biology of Habitability’ published in volume 16, issue 1 of Astrobiology, pages 7-22.
Chopra and Lineweaver specifically state that: ‘If life emerges on a planet, it only rarely evolves quickly enough to regulate greenhouse gases and albedo, thereby maintaining surface temperatures compatible with liquid water and habitability.’
Enter, ‘The Great Filter.’
The Great Filter:
The ‘Great Filter’ put simply, is Fermi’s original hypothesis that “technological civilization doesn’t last long enough for [interstellar flight] to happen.” While humans have already passed one of more ‘Gaian Bottlenecks’ – mass extinction events in our biological lineage – it is less likely we have already survived the ‘Great Filter.’ As such, geophysicist James Kasting – among those cited by Chopra and Lineweaver in their paper – when interviewed on the question of ‘Gaian Bottlenecks’ by online magazine www.inverse.com, stated: “I think climate change could be a Great Filter for us.”
Read more: ‘Failure to Find Aliens Means We’re in Safe Space’ – Inverse Science
Herein lies the foundation for answering our original three questions:
Why are we here?
Why are we alone?
And how long have we got to live?
Chemistry and Carbon:
The periodic table maps the structures and properties of all 118 known elements. Carbon in particular is special in that its existence answers our biggest questions.
We use carbon a thousand different ways every day. It’s in rubber, asphalt, diamonds, sugar, wax, oil, plastic, coal, limestone, carbohydrates, household gas in your cooktop, carbon monoxide, carbon dioxide, charcoal, methane, marble, graphite, chlorofluorocarbons hydrochlorofluorocarbons used in cosmetics, packing foam, aerosols, glue, paint, air conditioners, as well as all the plants and animals we use to build homes, and make clothing and food. Even the radioactive dating of fossils is based on measuring carbon in organic matter. Our entire civilisation, life as we know it, is based on the properties of carbon.
There are more than ten million different organic compounds known by chemists. Carbon is the only element that can form so many compounds – because each atom can form four bonds to other atoms, and because the carbon atom is small enough to fit into very large molecules. It’s also the fourth most common element in the universe and has the highest melting point – meaning it is least likely to change properties based on different temperatures as planets cool, oceans liquefy and atmospheres form.
If we think about these properties, carbon is a stand-out among all elements.
Let’s rank all elements in the Universe by:
- – Availability of multiple electrons to form bonds, being their valency,
- – Ability to bond with other elements using the ‘Pauling Scale’ of electronegativity (you can also use bond length but the results are the same),
- – Atomic radius, as the ability to fit into compact, complex molecules,
- – Thermal stability, being melting point (an element must not frequently transition states to support life), and
- – Abundance in the Universe
Carbon is:
- – 33rd on valency
- – 10th on radius
- – 12th on electronegativity
- – 1st on melting point
- – 4th on abundance
If you combine all these properties, no other element comes close.
|
PROPERTIES |
|
|
|
|
|
|
RANKS |
|
|
|
|
|
||
|
No. |
Sym |
Name |
v |
r |
e(X) |
mp |
a |
|
v |
r |
e(X) |
mp |
a |
SUM |
|
6 |
C |
carbon |
4 |
67 |
2.55 |
3500 |
0.005 |
|
33 |
10 |
12 |
1 |
4 |
60 |
|
16 |
S |
sulfur |
6 |
88 |
2.58 |
113 |
0.0005 |
|
6 |
15 |
11 |
83 |
10 |
125 |
|
14 |
Si |
silicon |
4 |
111 |
1.9 |
1410 |
0.0007 |
|
33 |
22 |
38 |
33 |
8 |
134 |
|
44 |
Ru |
ruthenium |
6 |
178 |
2.2 |
2250 |
4E-09 |
|
6 |
63 |
19 |
10 |
37 |
135 |
|
76 |
Os |
osmium |
6 |
185 |
2.2 |
3045 |
3E-09 |
|
6 |
67 |
19 |
4 |
39 |
135 |
|
78 |
Pt |
platinum |
6 |
177 |
2.28 |
1772 |
5E-09 |
|
6 |
61 |
17 |
17 |
34 |
135 |
|
34 |
Se |
selenium |
6 |
103 |
2.55 |
217 |
0.00000003 |
|
6 |
20 |
12 |
79 |
20 |
137 |
|
77 |
Ir |
iridium |
6 |
180 |
2.2 |
2410 |
2E-09 |
|
6 |
64 |
19 |
8 |
40 |
137 |
|
42 |
Mo |
molybdenum |
6 |
190 |
2.16 |
2617 |
5E-09 |
|
6 |
69 |
27 |
6 |
34 |
142 |
|
35 |
Br |
bromine |
7 |
94 |
2.96 |
-7 |
7E-09 |
|
1 |
18 |
8 |
90 |
31 |
148 |
|
45 |
Rh |
rhodium |
6 |
173 |
2.28 |
1966 |
6E-10 |
|
6 |
58 |
17 |
13 |
57 |
151 |
|
54 |
Xe |
xenon |
6 |
108 |
2.6 |
-112 |
0.00000001 |
|
6 |
21 |
10 |
94 |
22 |
153 |
|
74 |
W |
tungsten |
6 |
193 |
2.36 |
3410 |
5E-10 |
|
6 |
71 |
15 |
2 |
60 |
154 |
|
5 |
B |
boron |
3 |
87 |
2.04 |
2300 |
1E-09 |
|
52 |
14 |
30 |
9 |
51 |
156 |
|
52 |
Te |
tellurium |
6 |
123 |
2.1 |
449 |
9E-09 |
|
6 |
29 |
28 |
69 |
29 |
161 |
|
33 |
As |
arsenic |
5 |
114 |
2.18 |
816.8 |
8E-09 |
|
23 |
24 |
26 |
60 |
30 |
163 |
|
53 |
I |
iodine |
7 |
115 |
2.66 |
114 |
1E-09 |
|
1 |
25 |
9 |
82 |
51 |
168 |
|
46 |
Pd |
palladium |
4 |
169 |
2.2 |
1552 |
2E-09 |
|
33 |
55 |
19 |
22 |
40 |
169 |
|
7 |
N |
nitrogen |
3 |
56 |
3.04 |
-210 |
0.001 |
|
52 |
8 |
6 |
97 |
7 |
170 |
|
26 |
Fe |
iron |
3 |
156 |
1.83 |
1535 |
0.0011 |
|
52 |
44 |
43 |
25 |
6 |
170 |
|
75 |
Re |
rhenium |
7 |
188 |
1.9 |
3180 |
2E-10 |
|
1 |
68 |
38 |
3 |
68 |
178 |
|
82 |
Pb |
lead |
4 |
154 |
2.33 |
327 |
0.00000001 |
|
33 |
43 |
16 |
71 |
22 |
185 |
|
13 |
Al |
aluminium |
3 |
118 |
1.61 |
1050 |
0.00005 |
|
52 |
26 |
51 |
45 |
14 |
188 |
|
43 |
Tc |
technetium |
7 |
183 |
1.9 |
2200 |
0 |
|
1 |
65 |
38 |
11 |
73 |
188 |
|
8 |
O |
oxygen |
2 |
48 |
3.44 |
-218 |
0.01 |
|
79 |
6 |
3 |
98 |
3 |
189 |
|
24 |
Cr |
chromium |
6 |
166 |
1.66 |
1857 |
0 |
|
6 |
53 |
47 |
15 |
73 |
194 |
|
41 |
Nb |
niobium |
5 |
198 |
1.6 |
2468 |
2E-09 |
|
23 |
75 |
52 |
7 |
40 |
197 |
|
79 |
Au |
gold |
5 |
174 |
2.54 |
1064 |
6E-10 |
|
23 |
59 |
14 |
44 |
57 |
197 |
|
85 |
At |
astatine |
7 |
127 |
2.2 |
302 |
0 |
|
1 |
31 |
19 |
75 |
73 |
199 |
|
28 |
Ni |
nickel |
2 |
149 |
1.91 |
1453 |
0.00006 |
|
79 |
41 |
37 |
31 |
13 |
201 |
|
93 |
Np |
neptunium |
6 |
38 |
1.36 |
640 |
0 |
|
6 |
2 |
59 |
65 |
73 |
205 |
|
17 |
Cl |
chlorine |
5 |
79 |
3.16 |
-101 |
0 |
|
23 |
13 |
5 |
93 |
73 |
207 |
|
10 |
Ne |
neon |
0 |
38 |
3.98 |
-249 |
0.0013 |
|
100 |
2 |
1 |
100 |
5 |
208 |
|
40 |
Zr |
zirconium |
4 |
206 |
1.33 |
1852 |
0.00000005 |
|
33 |
81 |
61 |
16 |
17 |
208 |
|
58 |
Ce |
cerium |
4 |
67 |
1.12 |
795 |
0.00000001 |
|
33 |
10 |
84 |
61 |
22 |
210 |
|
29 |
Cu |
copper |
2 |
145 |
1.9 |
1083 |
0.00000006 |
|
79 |
37 |
38 |
42 |
16 |
212 |
|
36 |
Kr |
krypton |
2 |
88 |
3 |
-157 |
0.00000004 |
|
79 |
15 |
7 |
95 |
18 |
214 |
|
23 |
V |
vanadium |
5 |
171 |
1.63 |
1890 |
0 |
|
23 |
56 |
49 |
14 |
73 |
215 |
|
51 |
Sb |
antimony |
5 |
133 |
2.05 |
630 |
4E-10 |
|
23 |
32 |
29 |
68 |
65 |
217 |
|
27 |
Co |
cobalt |
4 |
152 |
1.88 |
1495 |
0 |
|
33 |
42 |
42 |
29 |
73 |
219 |
|
32 |
Ge |
germanium |
4 |
125 |
2.01 |
937 |
0 |
|
33 |
30 |
32 |
51 |
73 |
219 |
|
50 |
Sn |
tin |
4 |
145 |
1.96 |
232 |
4E-09 |
|
33 |
37 |
35 |
78 |
37 |
220 |
|
21 |
Sc |
scandium |
3 |
184 |
1.36 |
1539 |
0.00000003 |
|
52 |
66 |
59 |
24 |
20 |
221 |
|
83 |
Bi |
bismuth |
5 |
143 |
2.02 |
271 |
7E-10 |
|
23 |
36 |
31 |
76 |
55 |
221 |
|
1 |
H |
hydrogen |
1 |
53 |
2.2 |
-259 |
0.75 |
|
94 |
7 |
19 |
101 |
1 |
222 |
|
84 |
Po |
polonium |
6 |
135 |
2 |
254 |
0 |
|
6 |
33 |
33 |
77 |
73 |
222 |
|
18 |
Ar |
argon |
0 |
71 |
3.19 |
-189 |
0.0002 |
|
100 |
12 |
4 |
96 |
11 |
223 |
|
90 |
Th |
thorium |
4 |
156 |
1.3 |
1750 |
4E-10 |
|
33 |
44 |
63 |
18 |
65 |
223 |
|
15 |
P |
phosphorus |
5 |
98 |
2.19 |
44 |
0 |
|
23 |
19 |
25 |
86 |
73 |
226 |
|
73 |
Ta |
tantalum |
5 |
200 |
1.5 |
2996 |
8E-11 |
|
23 |
76 |
56 |
5 |
72 |
232 |
|
22 |
Ti |
titanium |
4 |
176 |
1.54 |
1660 |
0 |
|
33 |
60 |
55 |
19 |
73 |
240 |
|
31 |
Ga |
gallium |
3 |
136 |
1.81 |
30 |
0.00000001 |
|
52 |
34 |
44 |
88 |
22 |
240 |
|
96 |
Cm |
curium |
4 |
145 |
1.3 |
1340 |
0 |
|
33 |
37 |
63 |
35 |
73 |
241 |
|
4 |
Be |
beryllium |
2 |
112 |
1.57 |
1278 |
1E-09 |
|
79 |
23 |
53 |
37 |
51 |
243 |
|
92 |
U |
uranium |
6 |
193 |
1.38 |
1132 |
2E-10 |
|
6 |
71 |
58 |
40 |
68 |
243 |
|
95 |
Am |
americium |
4 |
118 |
1.3 |
994 |
0 |
|
33 |
26 |
63 |
48 |
73 |
243 |
|
57 |
La |
lanthanum |
3 |
88 |
1.1 |
920 |
2E-09 |
|
52 |
15 |
85 |
53 |
40 |
245 |
|
25 |
Mn |
manganese |
4 |
161 |
1.55 |
1245 |
0 |
|
33 |
48 |
54 |
38 |
73 |
246 |
|
72 |
Hf |
hafnium |
4 |
208 |
1.3 |
2150 |
7E-10 |
|
33 |
83 |
63 |
12 |
55 |
246 |
|
91 |
Pa |
protactinium |
5 |
205 |
1.5 |
1568 |
0 |
|
23 |
78 |
56 |
21 |
73 |
251 |
|
102 |
No |
nobelium |
3 |
56 |
1.3 |
827 |
0 |
|
52 |
8 |
63 |
57 |
73 |
253 |
|
12 |
Mg |
magnesium |
2 |
145 |
1.31 |
639 |
0.0006 |
|
79 |
37 |
62 |
67 |
9 |
254 |
|
9 |
F |
fluorine |
1 |
42 |
3.98 |
-220 |
0 |
|
94 |
4 |
1 |
99 |
73 |
271 |
|
39 |
Y |
yttrium |
3 |
212 |
1.22 |
1523 |
7E-09 |
|
52 |
84 |
78 |
27 |
31 |
272 |
|
47 |
Ag |
silver |
2 |
165 |
1.93 |
962 |
6E-10 |
|
79 |
52 |
36 |
50 |
57 |
274 |
|
101 |
Md |
mendelevium |
3 |
161 |
1.3 |
1245 |
0 |
|
52 |
48 |
63 |
38 |
73 |
274 |
|
94 |
Pu |
plutonium |
6 |
177 |
1.28 |
640 |
0 |
|
6 |
61 |
73 |
65 |
73 |
278 |
|
81 |
Tl |
thallium |
3 |
156 |
1.62 |
303 |
5E-10 |
|
52 |
44 |
50 |
74 |
60 |
280 |
|
60 |
Nd |
neodymium |
3 |
206 |
1.14 |
1010 |
0.00000001 |
|
52 |
81 |
82 |
47 |
22 |
284 |
|
48 |
Cd |
cadmium |
2 |
161 |
1.69 |
321 |
2E-09 |
|
79 |
48 |
46 |
72 |
40 |
285 |
|
68 |
Er |
erbium |
3 |
226 |
1.24 |
1522 |
2E-09 |
|
52 |
90 |
76 |
28 |
40 |
286 |
|
49 |
In |
indium |
3 |
156 |
1.78 |
157 |
3E-10 |
|
52 |
44 |
45 |
81 |
67 |
289 |
|
66 |
Dy |
dysprosium |
3 |
228 |
1.22 |
1412 |
2E-09 |
|
52 |
92 |
78 |
32 |
40 |
294 |
|
86 |
Rn |
radon |
6 |
120 |
0 |
-71 |
0 |
|
6 |
28 |
96 |
92 |
73 |
295 |
|
98 |
Cf |
californium |
4 |
194 |
1.3 |
900 |
0 |
|
33 |
73 |
63 |
54 |
73 |
296 |
|
2 |
He |
helium |
0 |
31 |
0 |
-272 |
0.23 |
|
100 |
1 |
96 |
102 |
2 |
301 |
|
71 |
Lu |
lutetium |
3 |
217 |
1.27 |
1656 |
1E-10 |
|
52 |
85 |
74 |
20 |
70 |
301 |
|
64 |
Gd |
gadolinium |
3 |
233 |
1.2 |
1311 |
2E-09 |
|
52 |
96 |
80 |
36 |
40 |
304 |
|
30 |
Zn |
zinc |
2 |
142 |
1.65 |
420 |
0 |
|
79 |
35 |
48 |
70 |
73 |
305 |
|
20 |
Ca |
calcium |
2 |
194 |
1 |
839 |
0.00007 |
|
79 |
73 |
87 |
56 |
12 |
307 |
|
59 |
Pr |
praseodymium |
4 |
247 |
1.13 |
935 |
2E-09 |
|
33 |
99 |
83 |
52 |
40 |
307 |
|
62 |
Sm |
samarium |
3 |
238 |
1.17 |
1072 |
5E-09 |
|
52 |
97 |
81 |
43 |
34 |
307 |
|
69 |
Tm |
thulium |
3 |
222 |
1.25 |
1545 |
1E-10 |
|
52 |
87 |
75 |
23 |
70 |
307 |
|
100 |
Fm |
fermium |
3 |
231 |
1.3 |
1527 |
0 |
|
52 |
94 |
63 |
26 |
73 |
308 |
|
67 |
Ho |
holmium |
3 |
226 |
1.23 |
1470 |
5E-10 |
|
52 |
90 |
77 |
30 |
60 |
309 |
|
80 |
Hg |
mercury |
2 |
171 |
2 |
-39 |
1E-09 |
|
79 |
56 |
33 |
91 |
51 |
310 |
|
87 |
Fr |
francium |
3 |
42 |
0 |
27 |
0 |
|
52 |
4 |
96 |
89 |
73 |
314 |
|
99 |
Es |
einsteinium |
4 |
228 |
1.3 |
860 |
0 |
|
33 |
92 |
63 |
55 |
73 |
316 |
|
97 |
Bk |
berkelium |
4 |
253 |
1.3 |
986 |
0 |
|
33 |
100 |
63 |
49 |
73 |
318 |
|
65 |
Tb |
terbium |
3 |
225 |
0 |
1360 |
5E-10 |
|
52 |
89 |
96 |
34 |
60 |
331 |
|
89 |
Ac |
actinium |
3 |
200 |
1.1 |
1050 |
0 |
|
52 |
76 |
85 |
45 |
73 |
331 |
|
70 |
Yb |
ytterbium |
3 |
222 |
0 |
824 |
2E-09 |
|
52 |
87 |
96 |
58 |
40 |
333 |
|
38 |
Sr |
strontium |
2 |
219 |
0.95 |
769 |
0.00000004 |
|
79 |
86 |
89 |
62 |
18 |
334 |
|
55 |
Cs |
caesium |
2 |
161 |
0.79 |
321 |
2E-09 |
|
79 |
48 |
95 |
72 |
40 |
334 |
|
61 |
Pm |
promethium |
3 |
205 |
0 |
1100 |
0 |
|
52 |
78 |
96 |
41 |
73 |
340 |
|
3 |
Li |
lithium |
1 |
167 |
0.98 |
180 |
6E-09 |
|
94 |
54 |
88 |
80 |
33 |
349 |
|
11 |
Na |
sodium |
1 |
190 |
0.93 |
98 |
0.00002 |
|
94 |
69 |
90 |
84 |
15 |
352 |
|
56 |
Ba |
barium |
2 |
253 |
0.89 |
725 |
0.00000001 |
|
79 |
100 |
92 |
63 |
22 |
356 |
|
63 |
Eu |
europium |
3 |
231 |
0 |
822 |
5E-10 |
|
52 |
94 |
96 |
59 |
60 |
361 |
|
88 |
Ra |
radium |
2 |
205 |
0.9 |
700 |
0 |
|
79 |
78 |
91 |
64 |
73 |
385 |
|
37 |
Rb |
rubidium |
1 |
265 |
0.82 |
39 |
0.00000001 |
|
94 |
102 |
93 |
87 |
22 |
398 |
|
19 |
K |
potassium |
1 |
243 |
0.82 |
64 |
0 |
|
94 |
98 |
93 |
85 |
73 |
443 |
Carbon loves to bond with other elements, is capable of forming complex proteins, is abundant, and thermally stable.
Also:
(a) Heavier elements than those in the periodic table may exist (i.e.: having more than 118 protons) in the core of some stars, however they are highly unstable outside of stars.
(b) Carbon is marginally too heavy to have formed immediately during the Big Bang. Rather, carbon atoms are formed inside stars in a process called ‘stellar nucleosynthesis.’ More precisely, the rules that govern matter – the arrangement of electrons in shells around similar numbers of protons and neutrons – were defined during the Big Bang. Carbon is a product of this structure.
(c) Another reason carbon is able to form so many organic compounds is ‘catenation’ – its ability to bond with itself in long chains. Owing to its valency of +/- 4 carbon – being a ‘Group 14’ element – will give or take electrons with other atoms and form multiple strong covalent bonds. It could be argued that this ‘tetravalence’ is even more important than total valency here. This would seem to be the case based on structures in terrestrial organic chemistry and greenhouse gases. However, replacing valence with tetravalence only serves to further exaggerate carbon’s uniqueness, while excluding sulfur. The existence of sulfur bacteria on earth is the best evidence of an alternative biochemistry. Also, including electronegativity in the ranking compensates somewhat for the use of total valence.
(d) The overall ranking of sulfur and silicon does not confirm their existence as alternative biochemistries given the large delta below carbon. However, it is interesting to note that sulfur bacteria already exist on earth, although still reliant on the presence of carbon. Silicon is also often proposed as a theoretical alternative chemistry due to catenation (above). Other alternatives to these are cosmically rare or exist at narrow temperature ranges. As such, the results of this ranking seem to be supported by other evidence. Ruthenium is also used in anti-cancer medication owing to it’s ability to bond with DNA and in photovoltaic cells to improve sensitivity to solar radiation.
(e) Even in these alternative biochemistries the central principle of the Carbon Paradox may also be upheld as sulfur also forms potent greenhouse gases and silane has similar properties to methane.
(f) The ‘overall rank’ of elements is based on the sum of rankings of individual properties. Experimenting with products and means only serves to further exaggerate carbon’s uniqueness, and using Tuples and Euclidean vectors to derive overall rank – given we are ranking a uniform number of properties in a uniform list of elements – proves redundant.
(g) In the graph shown in the video the sum of rankings of the elements is inverted to create a point system, scoring carbon relative to other elements – where a higher score indicates suitability for the formation of life.
Self-Replication:
Two years ago Jeremy England, a 31-year-old assistant professor at the Massachusetts Institute of Technology, derived a mathematical formula that shows that if you take carbon – in the presence of other key elements, and shine sunlight on it, it will inevitably form complex proteins to dissipate heat, based on the second law of thermodynamics:
These proteins eventually form cells which eventually evolve the behaviours we know as ‘life.’ In other words, life is an inevitable arrangement of carbon in concert with other light elements.
That’s why we are here.
But there’s a twist.
WHY ARE WE ALONE?
Something has stopped intelligent life from propagating throughout the universe, even though – statistically – it as good as certainly should have happened long ago. So why – if life is inevitable – isn’t it? This brings us back to the ‘Great Filter.’ Something stops life from getting past a certain point. The answer is surprisingly simple.
Carbon, for the same reasons it is the only source of life, is also deadly. We don’t have to look very far for an example.
Our twin planet Venus – right next door – has similar properties as Earth, is roughly the same size, shares a similar chemical composition and orbits the same sun. By any logic, life should have evolved there as well. But the atmosphere on Venus is 96.5% carbon dioxide.
|
|
VENUS |
EARTH |
RATIO |
|
RADIUS: |
6,052 km |
6,371 km |
95% |
|
MASS: |
4,867,500,000 Tt |
5,972,370,000 Tt |
82% |
|
AV. ORBIT: |
108,208,000 km |
149,598,023 km |
72% |
|
YEAR: |
224.7 days |
365 days |
62% |
|
GRAVITY: |
8.87 m/s² |
9.8 m/s² |
91% |
|
AGE: |
4.6 bn yrs |
4.543 bn yrs |
101% |
|
PLANETARY TEMP: |
232 K |
255 K |
91% |
|
TOTAL CO2: |
4.1 x 10e23 gm |
= |
|
|
v |
|
|
|
|
– ATMOSPHERE: |
4.1 x 10e23 gm |
1.4 x 10e20 gm |
292857% |
|
– FOSSIL FUELS: |
0 |
7 x 10e22 gm |
0% |
|
– CARBONATE ROCKS: |
0 |
3 x 10e23 gm |
0% |
|
– BIOSPHERE: |
0 |
10e19 gm |
0% |
|
– ATMOSPHERE: |
0 |
2.4 x 10e18 gm |
0% |
|
– OCEANS |
0 |
1.3 x 10e20 gm |
0% |
|
|
|
|
|
|
OBSERVED TEMP: |
735 K |
288 K |
255% |
As shown in the above table, all of Venus’ surface carbon is trapped in the atmosphere rather than in organic matter, oceans, rocks or soils. The greenhouse effect of so much CO2 in the atmosphere gives Venus an average atmospheric temperature of 462 degrees Celsius (864 degrees Fahrenheit) not only making it inhospitable to life but also evaporating its oceans and streams.
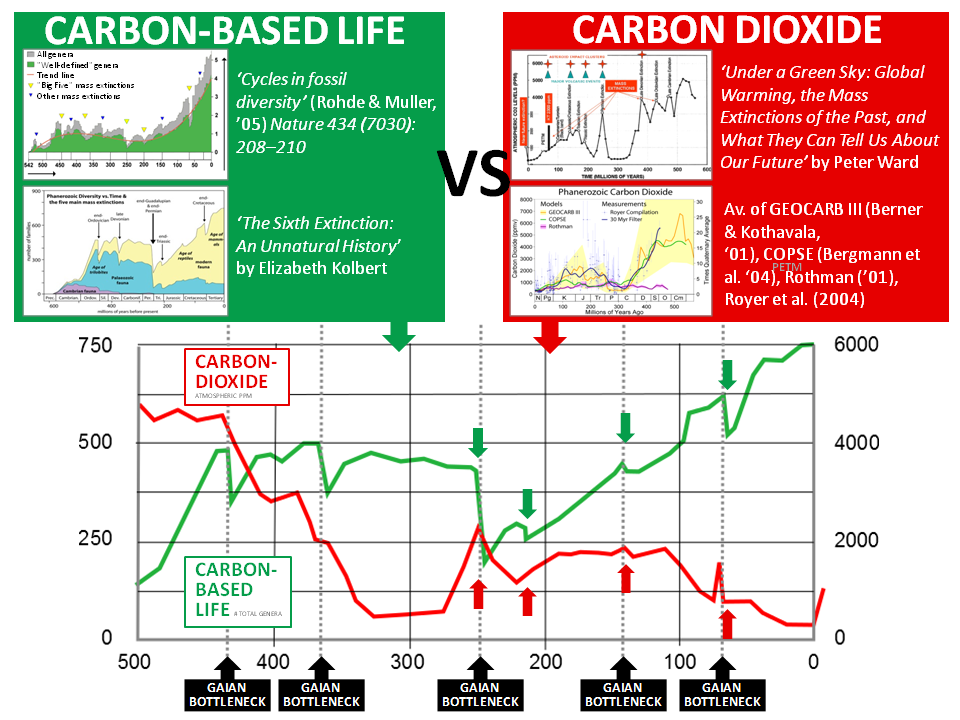
Graphing Life vs CO2:
Several near-miss mass extinction events have occurred in earth’s history. One event in particular very nearly ended life on earth. To map the inverse correlation between the number of surface organisms and atmospheric carbon, total genera was used from ‘Cycles in fossil diversity’ by Robert A. Rohde and Richard A. Muller of the Department of Physics and Lawrence Berkeley Laboratory, University of California – published in March 2005 in ‘Nature’ issue 434 (7030), pages 208–210. This chart shows fluctuations in diversity of marine fossil by genera for the past 542 million years, and has a high correlation with ‘A Kinetic Model of Phanerozoic Taxonomic Diversity. III. Post-Paleozoic Families and Mass Extinctions’ by J. John Sepkoski, Jr. first published in ‘Paleobiology’ Vol. 10, No. 2 (Spring, 1984), pp. 246-267
To illustrate the inverse correlation, and combining multiple references, I plotted the 30 million year filtered average of:
– Peter Ward’s ‘Atmospheric CO2 550 million years ago to the present’ from ‘Under a Green Sky’ published by Harper Collins in 2007,
– data from GEOCARB III by Robert A. Berner And Zavareth Kothavala, published in the American Journal of Science, Vol. 301, February, 2001, P. 182–204,
– ‘COPSE: A new model of biogeochemical cycling over Phanerozoic time’ by Noam M. Bergman, Timothy M. Lenton and Andrew J. Watson published in the American Journal of Science in May 2004, vol. 304 no. 5, pages 397-437,
– 2001 data from Daniel H. Rothman in the Proceedings of the National Academy of Sciences USA vol. 98 no. 8, pages 4305–4310, and
– ‘CO2 as a primary driver of Phanerozoic climate’ by Dana L. Royer, Robert A. Berner, Isabel P. Montañez, Neil J. Tabor and David J. Beerling published in GSA Today, volume 14 no. 3, p. 4-10.
Carbon not only inevitably forms life, but it also forms the most common greenhouse gases – CO2 and Methane – heating planets to the point where life is inevitably destroyed. This same correlation also demonstrates the feedback loop between extinction and the creation of fossil fuels. For intelligent life to form, it must evolve over hundreds of millions of years. Periodic ‘Gaian Bottlenecks’ ensure fuel exists for an advanced species to inevitably set about it’s own destruction.
As I described in my article ‘Elon Mustn’t: Why We Shouldn’t Go To Mars‘, you can trace the origins of humans back to cynodonts that survived the End Permian Mass Extinction 235 million years ago. In this same mass extinction the oil was formed by which we now fuel our own demise.
All of those millions of generations of billions of species, when they died, became coal, natural gas, and oil. For human civilisation to have developed to the point we are able to change our atmosphere, we had to harness primitive carbon stores. We first discovered and mastered fire 125,000 years ago and the moment we began burning wood, as that first whiff of greenhouse gas went skyward, we set a doomsday clock ticking.
With time, human’s advanced from wood to coal, coal to oil, and oil to natural gas – burning the same carbon that once formed life. Adequate stores of successive fuels tipped like dominoes. We can now trace the isotopes of carbon, namely carbon 13, in the atmosphere, to prove that the carbon we have burned is causing global warming.
Every life form that ever existed anywhere in the Universe would have faced exactly the same challenge. As surely as carbon forms life, it cascades into this inevitable pattern. Once CO2 levels in the atmosphere reach a tipping point – as we saw during the End Permian Mass Extinction 260 million years ago – rising ocean temperatures trigger the thaw of tundra, the death of jungles, and the release of deep ocean sediments called ‘Clathrates’. These kick the temperature up another 20 degrees as we also saw in the End Permian mass extinction. The only way the temperature declined is that enough algae survived in our oceans to slowly reabsorb that carbon, otherwise Earth would now look like Venus – hundreds of degrees hotter, and devoid of life.
That is why we are alone.
Just as inevitable as life, is it’s extinction – thanks to the very same properties of one critical element.
And that is the ‘Carbon Paradox.’ Carbon cause life, but is also the reason we haven’t encountered it elsewhere in the Universe.
Which brings us to the third question…
HOW LONG HAVE WE GOT TO LIVE?
In 1964, the Soviet astronomer Nikolai Kardashev developed what is now called the Kardashev scale, classifying humans as being at the very beginning of the scale – emerging ‘Type I’ – based on our energy sources. For any life form to survive long enough to travel the stars, it would have to achieve ‘Type 2’ – harnessing the energy of its sun. As such, our species is now at a pivotal turning point.
In the very same instant we have come to realise our predicament, it is almost too late. Our current rate of releasing carbon into the atmosphere is the fastest it has been in 66 million years, and faster than the period leading into the End-Permian mass extinction. Temperatures are predicted to rise in the next Century 20 times faster than at any other time in the past 2 million years.
Human civilisation is presented with a unique opportunity: will we learn to manage the atmosphere of our planet, and harness the Great Filter? Based on the work of Fermi, Drake, Frank, Sullivan, Bracewell, von Neumann, Tipler, Sagan, Newman and hundreds of other scientists, it is evident that no other species has successfully navigated this transition.
If we succeed, we may well be the species that explored the entire Universe in the next 4 million years.
If we do not, we will vanish without a trace taking all known life with us.
Meanwhile, across the Universe, the same inevitably opera plays out again and again, a trillion times over, and will until a species eventually thwarts the #carbonparadox.
PLEASE COMMENT
Categories: Featured.
Follow responses: RSS 2.0
Both comments and pings are currently closed.
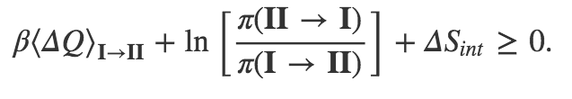
Sensational article (and video)!
I haven’t seen `the Carbon Paradox’ framed up and summarized this well anywhere before! – Truly terrific stuff.
(And, as a rural firefighter, I hope Trump and his climate-science-deniers all read and watch it… preferably on a continuous loop, until it sinks in for them 🙂
Thanks JT! It may be a little too long for certain people.
Awesome Blogpost Thanks for sharing.
[…] See also The Carbon Paradox – Marcus Gibson: […]
Saved as a favorite, I really enjoy your blog!
Great article and summary of carbon and its implications for civilisation and understanding of the Earth-life system. I would like to talk to you about the carbon paradox….
Thanks, D.C.
Everything is very open with a very clear description of the issues.
It was truly informative. Your website is very
helpful. Many thanks for sharing!
It’s a pity you don’t have a donate button! I’d
without a doubt donate to this excellent blog! I guess for now i’ll settle for book-marking and adding your RSS feed to my Google account.
I look forward to brand new updates and will share this website with my Facebook group.
Chat soon!
Keep this going please, great job!